
تست سایش | Abrasion Test
#تست سایش#آزمون سایش#آزمایش سایش#abrasion test#ابریشن تست#تست ابریشن#jsj shda
آزمون سایش | Abrasion Test
تست ساش به دو روش سایش پین بر روی دیسک و تست سایش خراشان و به دو صورت چرخشی یا رفت و برگشتی انجام می شود. با کمک آزمایش سایش می توان مقاومت سایشی قطعات را قبل از تولید یا به کارگیری بررسی کرد. آزمون سایش یک نوع نوع تست شبیه سازی محسوب می شود و جزء الزامات فلزات، کامپوزیت ها، سرامیک ها و پوشش های ضخیم جوشکاری شده می باشد.
در مجموعه آزمایشگاه های سنجش و پایش کیاژن فارما، تست سایش معمولا به منظور آنالیز مقاومت قطعات فلزی در برابر سایش بکار می رود.
#تست سایش#آزمون سایش#آزمایش سایش#abrasion test#ابریشن تست#تست ابریشن#jsj shda
توضیحات محصول
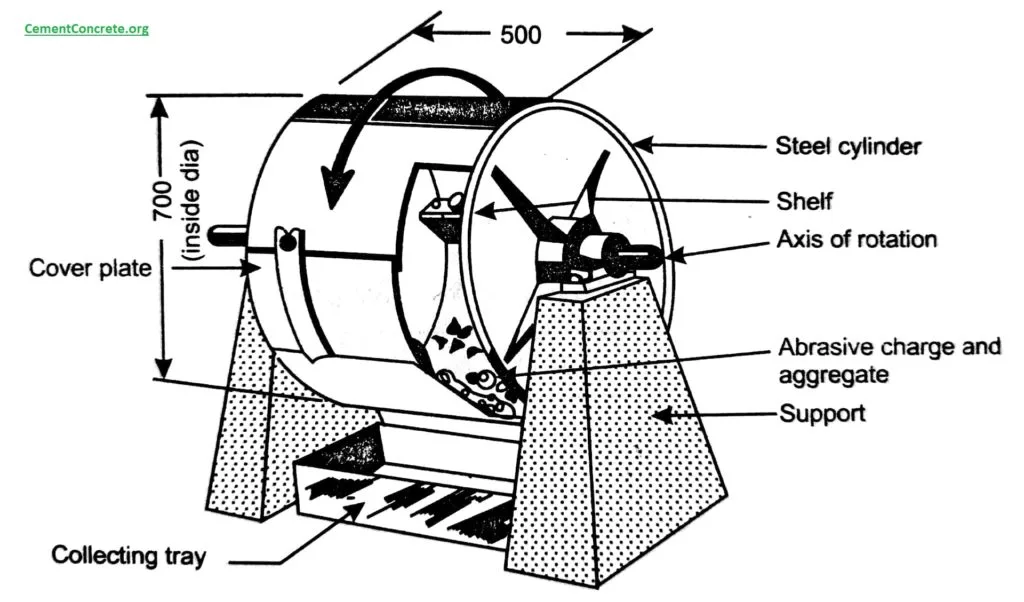

#تست سایش#آزمون سایش#آزمایش سایش#abrasion test#ابریشن تست#تست ابریشن#jsj shda
استاندارد های زیست سازگاری | References
ISO 10993-2: Biological Evaluation of Medical Devices – Animal welfare requirements.
ISO 10993-5: Biological Evaluation of Medical Devices – Tests for in vitro cytotoxicity.
ISO 10993-6: Biological Evaluation of Medical Devices – Tests for local effects after implantation.
ISO 10993-7: Biological Evaluation of Medical Devices – Ethylene oxide sterilization residuals.
ISO 10993-10: Biological Evaluation of Medical Devices – Tests for skin sensitization.
ISO 10993-11: Biological Evaluation of Medical Devices – Tests for systemic toxicity.
ISO 10993-12: Biological Evaluation of Medical Devices – Sample preparation and reference materials.
ISO 10993-22: Biological Evaluation of Medical Devices – Guidance on nanomaterials.
ISO 10993-23: Biological Evaluation of Medical Devices – Tests for irritation.
OECD Test No. 401: Guidelines for the Testing of Chemicals – Acute Oral Toxicity.
OECD Test No. 402: Guidelines for the Testing of Chemicals – Acute Dermal Toxicity.
OECD Test No. 403: Guidelines for the Testing of Chemicals – Acute Inhalation Toxicity.
OECD Test No. 404: Guidelines for the Testing of Chemicals – Acute Dermal Irritation/Corrosion.
OECD Test No. 405: Guidelines for the Testing of Chemicals – Acute Eye Irritation/Corrosion.
OECD Test No. 406: Guidelines for the Testing of Chemicals – Skin Sensitisation.
OECD Test No. 414: Guidelines for the Testing of Chemicals – Prenatal Developmental Toxicity Study.
OECD Test No. 416: Guidelines for the Testing of Chemicals – Two-Generation Reproduction Toxicity.
OECD Test No. 417: Guidelines for the Testing of Chemicals – Toxicokinetics.
OECD Test No. 424: Guidelines for the Testing of Chemicals – Neurotoxicity Study in Rodents.
OECD Test No. 426: Guidelines for the Testing of Chemicals – Developmental Neurotoxicity Study.
OECD Test No. 427: Guidelines for the Testing of Chemicals – Skin Absorption: In Vivo Method.
OECD Test No. 428: Guidelines for the Testing of Chemicals – Skin Absorption: In Vitro Method.
OECD Test No. 429: Guidelines for the Testing of Chemicals – Skin Sensitisation.
OECD Test No. 432: Guidelines for the Testing of Chemicals – In Vitro 3T3 NRU Phototoxicity Test.
OECD Test No. 440: Guidelines for the Testing of Chemicals – Uterotrophic Bioassay in Rodents.
OECD Test No. 441: Guidelines for the Testing of Chemicals – Hershberger Bioassay in Rats.
OECD Test No. 451: Guidelines for the Testing of Chemicals – Carcinogenicity Studies.
OECD Test No. 452: Guidelines for the Testing of Chemicals – Chronic Toxicity Studies.
OECD Test No. 456: Guidelines for the Testing of Chemicals – H295R Steroidogenesis Assay.
OECD Test No. 471: Guidelines for the Testing of Chemicals – Bacterial Reverse Mutation Test.
OECD Test No. 478: Guidelines for the Testing of Chemicals – Rodent Dominant Lethal Test.
OECD Test No. 484: Guidelines for the Testing of Chemicals – Genetic Toxicology: Mouse Spot Test.
OECD Test No. 489: Guidelines for the Testing of Chemicals – In Vivo Mammalian Alkaline Comet Assay.









نقد و بررسیها
هنوز بررسیای ثبت نشده است.