
تست سمیت ژنتیکی | HPRT
تست سمیت ژنتیکی سلول های پستانداران با استفاده از ژن HPRT
در مجموعه آزمایشگاه های سنجش و پایش کیاژن فارما، تست سمیت ژنتیکی در سلول های پستانداران (the in vitro mammalian cell gene mutation test) بر روی ژن های گزارشگر (forward mutations in reporter genes) مثل ژن hypoxanthine-guanine phosphoribosyl transferase gene (HPRT) انجام می شود.
تست HPRT جهت ارزیابی جهش رو به جلو در و همچنین ارزیابی مواردی مانند جایگزینی جفت باز (base pair substitutions)، تغییر چارچوب خوانش (frameshifts) و تشخیص جهش های ناشی از حذف و درج کوچک (small deletions and insertions) بواسطه تماس با لوازم و تجهیزات پزشکی (medical devices)، دارو (pharmaceuticals)، سم و آفت کش (pesticides)، محصولات آرایشی و بهداشتی (cosmetics)، مواد شیمیایی صنعتی (chemicals) و محصولات مصرفی (consumer products) انجام می شود.
تستهای سمیت ژنتیکی جزء الزامات سازمان ملی استاندارد، سازمان غذا و دارو و اداره کل تجهیزات پزشکی می باشد.
سلول هایی که بواسطه ماده آزمایش جهش زا، فعالیت آنزیمی HPRT آن دچار نقص شده است، به اثرات ضد رشد (cytostatic effects) تیوگوانین پورین purine analogue 6-thioguanine (TG) مقاوم هستند. در مقابل سلول های با سیستم آنزیمی فعال HPRT به TG (عامل ممانعت متابولیسم سلول و تقسیم سلول) حساس هستند. بنابراین سلول های موتانت قادر به تکثیر و تشکیل کلنی در حضور TG هستند درمقابل سلول های نرمال که حاوی آنزیم HPRT هستند قادر به تکثیر نیستند و کلنی تشکیل نخواهند داد.
توضیحات محصول
#آزمون hprt#jsj احقف#آزمون احقف#آزمون hprt#تست احقف#تست سمیت ژنی hprt#آزمون سمیت ژنی hprt#آزمون سمیت ژنتیکی hprt#تست سمیت ژنتیکی hprt#سمیت ژنتیکی اچ پی ار تی#hprt tests#سمیت ژنی اچ پی ار تی#آزمون سمیت ژنی اچ پی آر تی#تست سمیت ژنتیکی اچ پی آر تی#Salvage Pathway#de-novo#6-thioguanine (TG)#the in vitro mammalian cell gene mutation test#تست سمیت ژنی به روش hprt#تست سمیت ژنتیکی به روش hprt#تست hprt#آزمون hprt
تست سمیت ژنتیکی با استفاده از ژن HPRT
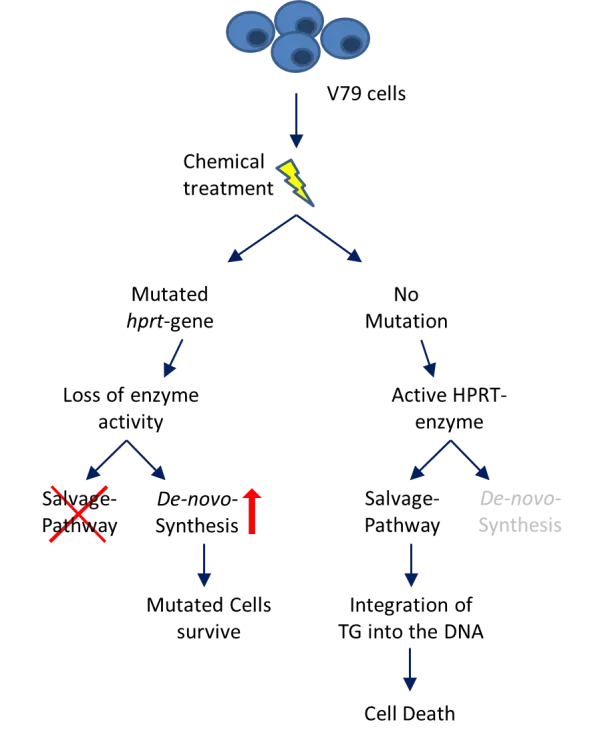
- آزمون HPRT به منظور تخمین اثر جهش زایی یک ماده، توانایی تشکیل کلنی لاین سلولی CHO با افزودن 6-thioguanine (TG) به محیط کشت سلول را بررسی می کند.
- مواد جهش زا باعث ایجاد جهش رو به جلو در ژن کد کننده آنزیم هیپوگزانتین-گوانین-فسفریبوزیل ترانسفراز (HPRT) می شود.
- جهش در این ژن منجر به بیان نادرست توالی آنزیم و در نتیجه از دست دادن عملکرد آن می شود که برای سنتز نوکلئوتیدها از طریق مسیر نجات (Salvage pathway) ضروری است.
- سلول ها می توانند نوکلئوتیدها را از طریق بیوسنتز de-novo یا از طریق مسیرهای بازیافت صرفه جویی در انرژی (Salvage Pathway) با استفاده از بازهای آزاد (مانند آدنین، گوانین) بدست آورند.
- سلولهای CHO با ژن hprt دستنخورده، آنالوگ پایه پورین سمی (TG) را از طریق مسیر نجات به DNA خود وارد میکنند که منجر به مهار متابولیسم سلولی و سمیت سلولی میشود.
- از دست دادن عملکرد آنزیم HPRT به دلیل جهش، منجر به افزایش سنتز de-novo می شود زیرا مسیر Salvage حذف می شود و سلولهای CHO آسیبدیده قادر به ترکیب TG سمی در DNA نیستند، بنابراین سلول های جهش یافته می توانند در حضور TG تکثیر شوند، در حالی که سلول های طبیعی که حاوی HPRT هستند، این گونه نیستند و ازبین خواهند رفت.
#آزمون hprt#jsj احقف#آزمون احقف#آزمون hprt#تست احقف#تست سمیت ژنی hprt#آزمون سمیت ژنی hprt#آزمون سمیت ژنتیکی hprt#تست سمیت ژنتیکی hprt#سمیت ژنتیکی اچ پی ار تی#hprt tests#سمیت ژنی اچ پی ار تی#آزمون سمیت ژنی اچ پی آر تی#تست سمیت ژنتیکی اچ پی آر تی#Salvage Pathway#de-novo#6-thioguanine (TG)#the in vitro mammalian cell gene mutation test#تست سمیت ژنی به روش hprt#تست سمیت ژنتیکی به روش hprt#تست hprt#آزمون hprt
#آزمون hprt#jsj احقف#آزمون احقف#آزمون hprt#تست احقف#تست سمیت ژنی hprt#آزمون سمیت ژنی hprt#آزمون سمیت ژنتیکی hprt#تست سمیت ژنتیکی hprt#سمیت ژنتیکی اچ پی ار تی#hprt tests#سمیت ژنی اچ پی ار تی#آزمون سمیت ژنی اچ پی آر تی#تست سمیت ژنتیکی اچ پی آر تی#Salvage Pathway#de-novo#6-thioguanine (TG)#the in vitro mammalian cell gene mutation test#تست سمیت ژنی به روش hprt#تست سمیت ژنتیکی به روش hprt#تست hprt#آزمون hprt
استاندارد های زیست سازگاری | References
ISO 10993-2: Biological Evaluation of Medical Devices – Animal welfare requirements.
ISO 10993-5: Biological Evaluation of Medical Devices – Tests for in vitro cytotoxicity.
ISO 10993-6: Biological Evaluation of Medical Devices – Tests for local effects after implantation.
ISO 10993-7: Biological Evaluation of Medical Devices – Ethylene oxide sterilization residuals.
ISO 10993-10: Biological Evaluation of Medical Devices – Tests for skin sensitization.
ISO 10993-11: Biological Evaluation of Medical Devices – Tests for systemic toxicity.
ISO 10993-12: Biological Evaluation of Medical Devices – Sample preparation and reference materials.
ISO 10993-22: Biological Evaluation of Medical Devices – Guidance on nanomaterials.
ISO 10993-23: Biological Evaluation of Medical Devices – Tests for irritation.
OECD Test No. 401: Guidelines for the Testing of Chemicals – Acute Oral Toxicity.
OECD Test No. 402: Guidelines for the Testing of Chemicals – Acute Dermal Toxicity.
OECD Test No. 403: Guidelines for the Testing of Chemicals – Acute Inhalation Toxicity.
OECD Test No. 404: Guidelines for the Testing of Chemicals – Acute Dermal Irritation/Corrosion.
OECD Test No. 405: Guidelines for the Testing of Chemicals – Acute Eye Irritation/Corrosion.
OECD Test No. 406: Guidelines for the Testing of Chemicals – Skin Sensitisation.
OECD Test No. 414: Guidelines for the Testing of Chemicals – Prenatal Developmental Toxicity Study.
OECD Test No. 416: Guidelines for the Testing of Chemicals – Two-Generation Reproduction Toxicity.
OECD Test No. 417: Guidelines for the Testing of Chemicals – Toxicokinetics.
OECD Test No. 424: Guidelines for the Testing of Chemicals – Neurotoxicity Study in Rodents.
OECD Test No. 426: Guidelines for the Testing of Chemicals – Developmental Neurotoxicity Study.
OECD Test No. 427: Guidelines for the Testing of Chemicals – Skin Absorption: In Vivo Method.
OECD Test No. 428: Guidelines for the Testing of Chemicals – Skin Absorption: In Vitro Method.
OECD Test No. 429: Guidelines for the Testing of Chemicals – Skin Sensitisation.
OECD Test No. 432: Guidelines for the Testing of Chemicals – In Vitro 3T3 NRU Phototoxicity Test.
OECD Test No. 440: Guidelines for the Testing of Chemicals – Uterotrophic Bioassay in Rodents.
OECD Test No. 441: Guidelines for the Testing of Chemicals – Hershberger Bioassay in Rats.
OECD Test No. 451: Guidelines for the Testing of Chemicals – Carcinogenicity Studies.
OECD Test No. 452: Guidelines for the Testing of Chemicals – Chronic Toxicity Studies.
OECD Test No. 456: Guidelines for the Testing of Chemicals – H295R Steroidogenesis Assay.
OECD Test No. 471: Guidelines for the Testing of Chemicals – Bacterial Reverse Mutation Test.
OECD Test No. 478: Guidelines for the Testing of Chemicals – Rodent Dominant Lethal Test.
OECD Test No. 484: Guidelines for the Testing of Chemicals – Genetic Toxicology: Mouse Spot Test.
OECD Test No. 489: Guidelines for the Testing of Chemicals – In Vivo Mammalian Alkaline Comet Assay.









نقد و بررسیها
هنوز بررسیای ثبت نشده است.